Liquid Crystals
 An introduction to liquid crystals, suitable for schools, is available to download from here. Introduction (PDF). This is written in two chapters, in PDF format, which can be viewed using Acrobat Reader.
An introduction to liquid crystals, suitable for schools, is available to download from here. Introduction (PDF). This is written in two chapters, in PDF format, which can be viewed using Acrobat Reader.
A liquid crystal is, as the name suggests, a state of matter intermediate between a 'normal' liquid and a solid. Liquid crystal phases are formed from geometrically anisotropic molecules - usually this means they are cigar shaped, though other shapes are possible. In a liquid crystal phase, the molecules have a certain degree of order. In the simplest case, the Nematic phase, the molecules generally point in the same direction but still move around with respect to one another as would be expected in a liquid. It is this type of liquid crystal that is used in liquid crystal display devices (LCDs). The most common LCD uses nematic liquid crystals, and is known as a twisted nematic (TN) device.
The nematic phase - note that the molecules are rod-shaped and all point in approximately the same direction.
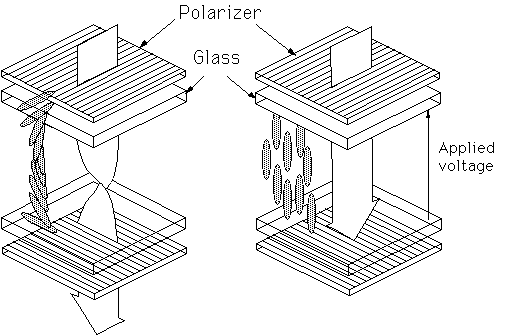
The operation of a TN device is shown here. The thickness of the liquid crystal layer within the device is approximately 10 microns. The device is constructed so that without a voltage applied, the liquid crystal molecules adopt a twisted structure, as shown. This twisted configuration will guide plane polarised light to pass through the bottom polariser. On application of a sufficiently large voltage, the molecules rotate to point in the direction of the electric field, destroying the twisted structure and hence the ability to guide light. The polarised light travelling through the device can therefore not pass the bottom polariser. The device is thus a simple light shutter; with no voltage applied, it transmits light, whilst when a small voltage is applied (usually ~1V), light is no longer transmitted.
A further range of liquid crystal phases are possible if the molecules that form them are chiral (this means that the mirror image of the molecule cannot be superimposed upon itself - rather like the distinction between left and right-handed screw threads). The molecular chirality can cause the liquid crystal phase to adopt a helicoidal (spiral) structure. In the simplest case, a chiral nematic (or cholesteric) phase, the helicoidal structure often has a periodicity approximately the same as the wavelength of visible light, so that colours are brightly reflected from these materials. Cholesteric liquid crystals are commonly used in thermometers, where a change in temperature causes a change in the pitch of the spiral structure and therefore a variation in the colour of the reflected light.
- The cholesteric structure is shown schematically here. Note that the average direction in which the liquid crystal molecules point traces out a spiral as you move through the structure.
The next most ordered phases are the so-called smectic phases; here, in addition to the molecules pointing in a preferred direction, they also form layers.
- The simplest smectic structure, the smectic-A, is shown here. The molecules lie in layers and point in the direction of the layer normal. the molecules' positions are random within the layers.
 Because it is possible to have various degrees of order within a layer different types of smectic phases may form. Some have the molecules perpendicular to the layers, some have the molecules in the layers tilted at some angle. If the molecules are tilted between layers and the molecules that form them are chiral, then it is possible for the tilt of molecules to precess between layers so again, a spiral is formed as you move from layer to layer. Chirality in liquid crystals is a subject that is currently of great interest as it can lead to the formation of 'frustrated' phases. These are phases which are stabilised by periodic arrays of defects that may be linear, cubic, hexagonal, or even tetragonal. In these frustrated phases, the energy cost of forming defects is balanced by increased twist in the structure.
Because it is possible to have various degrees of order within a layer different types of smectic phases may form. Some have the molecules perpendicular to the layers, some have the molecules in the layers tilted at some angle. If the molecules are tilted between layers and the molecules that form them are chiral, then it is possible for the tilt of molecules to precess between layers so again, a spiral is formed as you move from layer to layer. Chirality in liquid crystals is a subject that is currently of great interest as it can lead to the formation of 'frustrated' phases. These are phases which are stabilised by periodic arrays of defects that may be linear, cubic, hexagonal, or even tetragonal. In these frustrated phases, the energy cost of forming defects is balanced by increased twist in the structure.
Diagrams and photographs (viewed with a polarising microscope) of some liquid crystal phases are shown below.

- This photograph of a cholesteric liquid crystal shows what happens when two materials with a different sense of twist meet. Towards the centre of the picture, the two opposite twist senses compete and pitch of the helix becomes larger and larger. In the very centre of the picture the helix is completely unwound - this is known as 'compensated'.

- A photograph of a Blue Phase. Note the regular shapes of the blue phase domains reflects the cubic structure of the phase.
Most liquid crystals that react to heat, thermotropic liquid crystals, can be thought of as roughly having a cigar shape. In practice there is usually a rigid central section to the molecule (commonly including biphenyl units) with flexible alkyl or alkoxy chains on either end. The molecule may also contain units that result in permenant electronic dipoles, facilitating the interaction with electric fields in devices. The interaction of light with liquid crystal molecules depends on it's polarisation. If the light interacts primarily with the long axis of the molecule, it will experience a different refractive index than it would if it interacted with the molecular short axis - a property known as birefringence. Most liquid crystal devices incorporate polarisers and are designed so that the liquid crystal within the device lies in a specific direction, so that their optical response can be optimised when a voltage is applied to the device. Much research has taken place in making faster, smaller and low power consumption liquid crystal displays, suitable for use in portable computers, camcorders etc.
Although the liquid crystals that are associated with electronic devices all exhibit their liquid crystallinity as a function of temperature, and are exploited for their optical and electrical properties, another extremely important class of liquid crystals exist. Certain materials form ordered fluids when dissolved in a solvent (often water) and such systems are known as lyotropic liquid crystals. Materials that have this property are well known in nature and include DNA, certain viruses, soap, anti-asthmatic drugs etc. The liquid crystal phases that are exhibited depend on the concentration of the lyotropic solution as well as the temperature. A typical molecule that forms a lyotropic liquid crystal will have a hydrophilic part and a hydrophobic part. When the compound is placed in water, the hydrophilic ends will try to be near the water whilst the hydrophobic parts try to move away from it. The result is that at low concentrations micelles are formed. These are small spherical aggregates of molecules where the surface is formed from hydrophilic head groups and the centre contains the hydrophobic tails. As the concentration increases, different structures are formed, including tubes with, again, the head groups on the outside and the tails in the centre. Still higher concentrations of solute results in a layered structure layers of the molecules such that the structure is water-head-tail-tail-head-water and so on.
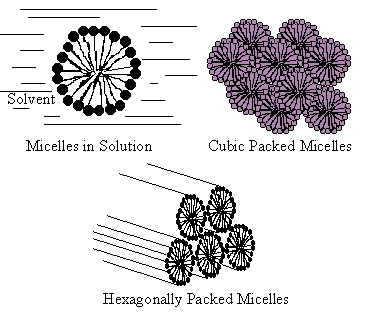
- Diagrams of some of the most common lyotropic phase structures.
Many interesting lyotropic liquid crystals are shaped like discs with groups on the edge that are hydrophilic or hydrophobic. These can stack in columns and show a different series of phases on when the concentration or temperatures are varied. Certain materials exhibit both thermotropic and lyotropic liquid crystal phases.
- Some of the discotic phases.
The area of liquid crystals is extremely broad. Some of the links shown on the Liquid Crystal Group home page will give more detailed descriptions of various kinds of liquid crystal phases, their chemistry, physics and applications. Happy surfing!
If you have any comments on our page then feel free to e-mail me at: Helen.Gleeson@manchester.ac.uk